Lodonal (TM)
Lodonal is currently approved in Nigeria for the treatment of HIV
The approval is for a one-day Immune System Regulator for the management of HIV/AIDS, which is based on the results of the Company’s 90-Day bridging trial in Nigeria that resulted in a 44% increase in CD4 Count versus an 11% increase for standard of care patients. Additionally, there was a reduction in opportunistic infections plus several Phase II multi-center, randomized studies that demonstrated improvements for patients treated with Lodonal when compared to placebo or standard of care.
EX-US Strategy: Nigerian Manufacturing

Nigerian Manufacturing Partner
Swiss Pharma Nigeria Limited (Swipha) is a subsidiary of Servier, a French Pharmaceutical Company that employs over 20,000 employees with nearly 3,000 focusing on Research and Development.
Servier acquired Swipha in 2017 to launch the presence of the French second biggest pharmaceutical company and extend their wide range of products to Nigerians.
Our origin is from a Swiss corporation (Roche Pharmaceutical) which was incorporated in 1976. In 2008 became the first pharmaceutical company in Nigeria to obtain ISO 9001 certification and in 2014 again became the first company in West Africa to obtain WHO GMP certification.
We have our manufacturing site in the heart of Lagos producing over 50 SKUs, and over 30 brands across different therapeutic categories such as Anti-diabetes, Anti-infectives, Anti-malarial, Central Nervous System and Cardio-vascular.
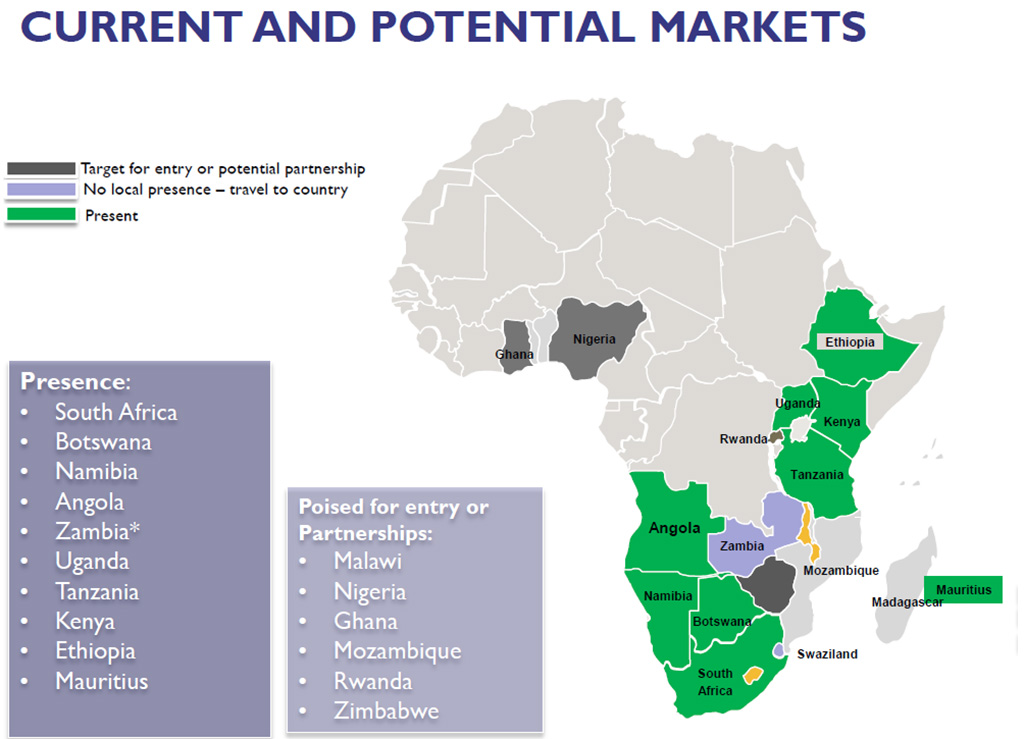
EX-US Strategy: African Continent CSO & Regulatory Group

One Partner Africa
One Partner Africa: Representing Biostax International to bring Lodonal™ and future molecules to Middle East & African markets (Including Indian ocean islands)
Contract Sales Team
- Syndicated Sales Personnel
- Exclusive Sales Teams
- Internal Salesforce
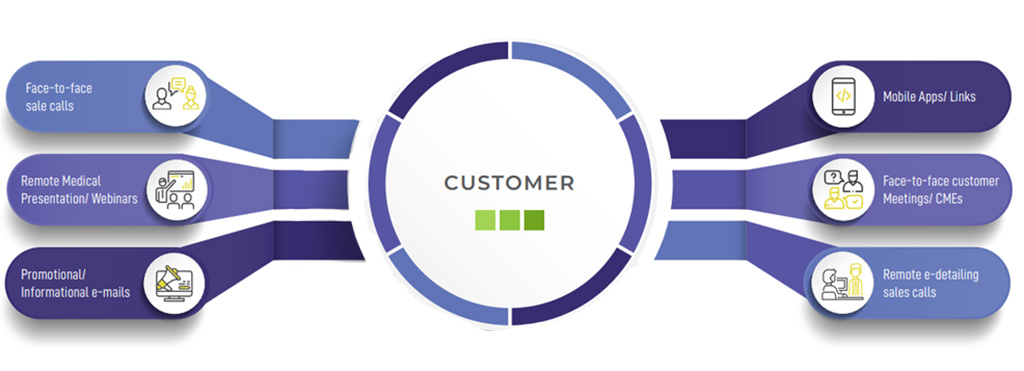
Marketing & Promotion
Develop marketing and promotional materials ensuring medical promotion to HCP’s / Key Account and Access activities to institutions and governments